Urea-Formaldehyde Resins
(Urea-methanal)
Properties
Urea-formaldehyde (UF) products (also called aminoplasts or carbamide-methanal) are highly crosslinked, semi-crystalline thermosetting plastics1. The UF resins are noted for their high strength, rigidity, cost effectiveness, and fast cure. Infact, they are some of the fastest curing resins available. At elevated temperatures, they can be cured in as little as two seconds.
A large portion of UF resins is consumed by the wood products industry. Infact, UF resins are the most popular adhesives for particleboards. The popularity of urea-formaldehyde resins as the main adhesive for wood products has several reasons, including low cost, ease of use under a variety of curing conditions, versatility, low cure temperature, resistance to mold formation, excellent thermal properties, lack of color of the cured product, and excellent water solubility of the (uncured) resin. A major drawback of urea- formaldehyde adhesives compared with other thermosetting wood adhesives, such as phenol-formaldehyde and polymeric diisocyanates, is the lack of moisture resistance especially at elevated temperatures. Furthermore, the reversal bond-forming reactions can lead to the release of formaldehyde.
Due to toxicity concerns from health and environmental agencies and customers (formaldehyde is a suspected carcinogen), the UF industry has developed new urea-formaldehyde resins with very low formaldehyde release. Two well known low emitting resin systems are Hexion's EcoBindTM and Arclin's E-Natural®. These resins were engineered to emit at or below naturally occuring levels while maintaining comparable performance.
Synthesis
Urea formaldehyde (UF) resins are primarily made up of urea and formaldehyde with formaldehyde acting as the crosslinker. The UF resins are formed in water at a pH above 7 at the start of the reaction, because the methylol derivatives that form in the first steps condense rapidly at acidic conditions:
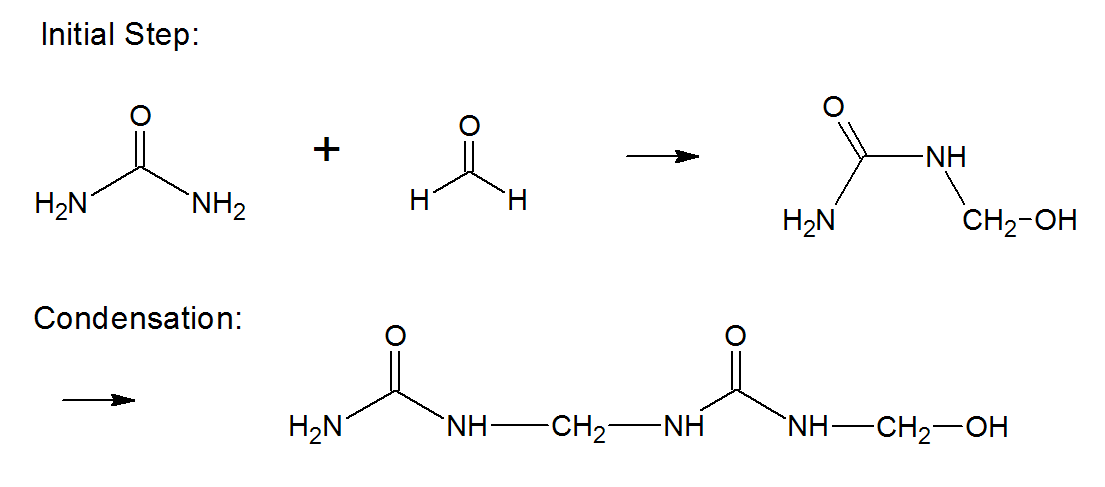
Under alkaline conditions, on the other hand, the condensaton is much slower and better to control. Instead of methylene linkages, dimethylene ether groups form:
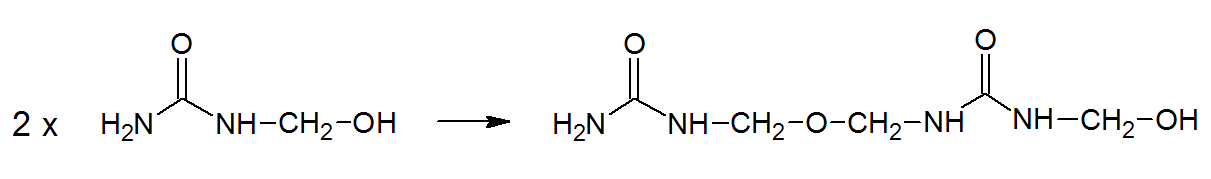
The condensed oligomers are water soluble and contain hemiformal groups. This mixture is further reacted under acidic conditions (usually at about pH 5) and by heating, which leads to the desired degree of condensation.
In the case of surface coatings, the UF resins are often modified to improve their solubility in solvent, for example, by reacting them with n-butyl alcohol (etherification) prior condensation (resin formation).
Commercial UF Resins
Major manufacturers of UF resins are Hexion, Arclin, Georgia-Pacific, Tembec, BASF, and Hexza.
Applications
Urea-formaldehyde resins find applications in adhesives, coatings, laminatings, and moulding compositions. More than 1 million metric tons of urea-formaldehyde (UF) resin is produced annually.2 This comprises about 80% of all amino resins produced worldwide. More than 70% of it is consumed by the wood industry for bonding particleboards (61%), medium-density fiberboard (27%), and hardwood plywood (5%). It is also used as an adhesive for decorative laminate applications (7%). Other applications include wet laid fiberglass mats, air filtration, coated and bonded abrasives.
Due to the lack of moisture resistance, UF wood adhesives are mainly used for the manufacture of wood products intended for interior use only.
1The reaction products of urea, melamine and related compounds such as dicyandiamide with fomaldehyde are called aminoplasts.
2The global formaldehyde market
for resins was worth USD 10.9 billion
in 2012 and is expected to reach USD 18.1 billion by 2018
Source: MRRSE (Market Research Reports Search Engine), October 21, 2015